Fungalpedia – Note 251, Thyrostroma
Thyrostroma Höhn.
Citation when using this entry: Aumentado et al. 2024 (in prep) – Fungalpedia, plant pathogens.
Index Fungorum, Facesoffungi, MycoBank, GenBank, Fig. 1
Classification: Botryosphaeriaceae, Botryosphaeriales, Incertae sedis, Dothideomycetes, Pezizomycotina, Ascomycota, Fungi
Thyrostroma was established by Höhnel (1911) and typified with T. compactum. This genus belongs to the Dothidotthiaceae family of the order Pleosporales within the Dothideomycetes class of Ascomycota (Hongsanan et al. 2020). Thyrostroma has previously been considered synonymous with several other genera, including Coryneum, Stegonsporium, Stigmina, Thyrococcum, Thyrostromella, and Wilsonomyces (Höhnel 1911, Morgan-Jones 1971, Sutton & Pascoe 1989). Previously, Thyrostroma was considered to be the asexual form of Dothidotthia owing to its hyphomycete state in culture (Phillips et al. 2008), but there has been no phylogenetic validation to support this connection. The incorporation of new morphological insights and phylogenetic examinations established that Thyrostroma and Dothidotthia species are not congeneric and should be retained as separate genera (Crous et al. 2016, Marin-Felix et al. 2017, Senwanna et al. 2019).
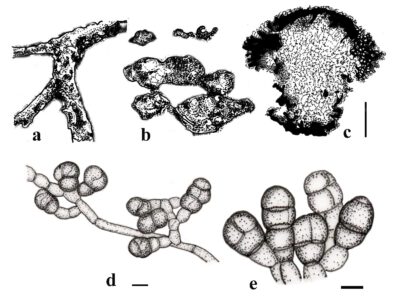
Thyrostroma is identified by its sporodochial conidiomata, which are punctiform and dark brown or black. The stroma are immersed to superficial, and brown. The conidiophores are brown, with a finely roughened surface, and are cylindrical to subcylindrical, with 1–3 septa. The conidiogenous cells are brown, sub-cylindrical, and finely roughened. Conidiogenous cells proliferate percurrently at the apex. The conidia are cylindrical, clavate, ellipsoid, or fusoid, pale to medium brown and have a smooth wall. Conidia have (1–) 4 transverse septa and can have 0 to 3 oblique or longitudinal septa. The conidial apex is rounded, whereas the base is truncated (Crous et al. 2016, Marin-Felix et al. 2017).
The taxonomy of Thyrostroma previously relied more on morphological characteristics when there were still no phylogenetic studies involved. Consequently, Thyrostroma grouped in a well-supported clade within the Dothidotthiaceae using LSU sequence data (Marin-Felix et al. 2017, Crous et al. 2019). To accurately identify genera and species, along with determining their taxonomic position, phylogenetic examinations using LSU, SSU, ITS, and tef1-α genetic markers were employed (Senwanna et al. 2019). Although a combined approach of LSU, SSU, ITS, and tef1-α genes sufficiently resolves species differentiation, a comparison of ITS and tef1-α gene regions indicates that most Thyrostroma species are not markedly distinct from each other. In light of this, Senwanna et al. (2019) proposed that rpb2 and tub2 genes are reliable markers for discerning species within Thyrostroma. Similarly, Jayawardena et al. (2020) reconstructed the phylogeny employing similar genetic markers and validated thirteen species, supporting the findings of Marin-Felix et al. (2017), Senwanna et al. (2019), and Hyde et al. (2020).
Thyrostroma species are pathogens, saprobes, or endophytes. As a pathogen, Thyrostroma is associated with canker, dieback, and leaf spots in terrestrial habitats (Yuan & Old 1990, Marin-Felix et al. 2017, Senwanna et al. 2019). Species of Thyrostroma have been recorded from various symptomatic plants viz., Cornus officinalis (Crous et al. 2016), Sambucus caerulea, Styphnolobium japonicum, Tilia spp. (Marin-Felix et al. 2017), and Ulmus spp. (Ellis 1971). Pathogenicity tests were conducted on Prunus spp., which produced circular to irregular lesions (Tovar-Pedraza et al. 2013, Nabi et al. 2018). A cross-infectivity study by Rasool et al. (2020) on Prunus spp. revealed that Thyrostroma isolates originating from cherries demonstrated the capability to infect apricots, but the isolates sourced from apricots and almonds did not exhibit cross-infectivity when subjected to other stone fruit hosts. Nevertheless, further studies are needed to underpin the host-specificity and pathogenic breadth of Thyrostroma.
Type species: Thyrostroma compactum (Sacc.) Höhn. 1911
Other species: (Species Fungorum – search Thyrostroma)
Figure 1– Thyrostroma tiliae (a-c) and Thyrostroma cornicola (d-e). a Canker symptoms. b Sporodochia on host surface. c Vertical section of sporodochium. d Conidiogenous cells and attachment of conidia. e. Conidia. Scale bars: c = 200 µm; d,e = 10 μm. Redrawn from Crous et al. (2016) and Senwanna et al. (2019).
References
Ellis MB. 1971 – Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew.
Yuan ZQ, Old KM. 1990 – A new species of Thyrostroma from Australia. Mycol Res, 94, 573–576.
Entry by
Herbert Dustin R. Aumentado, Center of Excellence in Fungal Research and School of Science, Mae Fah Luang University, Chiang Rai, Thailand
(Edited by Ruvishika S. Jayawardena & Kevin D. Hyde, Samaneh Chaharmiri-Dokhaharani, & Achala R. Rathnayaka)
Published online 20 May 2024