Fungalpedia – Note, 250 Pseudoseptoria
Pseudoseptoria Speg.
Citation when using this entry: Aumentado et al. 2024 (in prep) – Fungalpedia, plant pathogens.
Index Fungorum, Facesoffungi, MycoBank, GenBank, Fig 1.
Classification: Saccotheciaceae, Dothideales, Dothideomycetidae, Dothideomycetes, Pezizomycotina, Ascomycota, Fungi
Pseudoseptoria was introduced by Spegazzini (1910) to accommodate its type species Pseudoseptoria donacis. Wijayawardene et al. (2012) classified Pseudoseptoria within Ascomycota as genera incertae sedis, whereas Quaedvlieg et al. (2013) positioned Pseudoseptoria within Dothioraceae. However, Thambugala et al. (2014) and Wijayawardene et al. (2017) accepted Pseudoseptoria as a separate genus within Saccotheciaceae.
Pseudoseptoria is characterised by submerged, branched, septate, and brownish mycelium. Pycnidial conidiomata can be either solitary or arranged linearly. The conidiomata are immersed, brown, and globose, consisting of thin-walled walls made up of pale brown cells in an angular pattern. Notably, Pseudoseptoria has distinct central circular ostioles. The conidiogenous cells are separate, determinate, or indeterminate, hyaline, and smooth. The conidiogenous cells are ampulliform with a prominent cylindrical papilla and are falcate. Conidia are fusoid, hyaline, and aseptate, contain guttules, and have smooth, thin walls, with pointed rounded ends (Sutton 1980, Quaedvlieg et al. 2013).
Quaedvlieg et al. (2013) made a comprehensive revision of the Septoria and Septoria-like genera based on morphological observations and analyses of multiple genetic loci. Quaedvlieg et al. (2013) introduced two new species based on data derived from LSU, ITS, tef1-α, and rpb2 genes. Crous et al. (2017) conducted a phylogenetic assessment using only sequence data from LSU. While there are eight names listed in Species Fungorum (2023) under Pseudoseptoria with reported morphological characters, only three of these species have available molecular sequence data (P. collariana, P. donacis, and P. obscura). Similarly, Jayawardena et al. (2020), employing LSU, ITS, and rpb2 sequences, achieved congruent results and accepted three Pseudoseptoria species.
Pseudoseptoria species are mostly phytopathogens known to infect various members of the Poaceae, including Alopecurus pratensis, Arrhenatherum elatius, Arundo donax, Bromus spp., Dactylis glomerata, Danthonia spicata, Elymus alaskanus, Festuca rubra, Hordeum vulgare, Panicum virgatum, Phleum spp., Phragmites australis, and Poa spp. (Ginns 1986, Pennycook 1989, Gravert & Munkvold 2002, Dugan & Lupien 2003, Mulenko et al. 2008, Farr & Rossman 2023). Pathogenicity tests were conducted on Hordeum distichum (Carmona et al. 1996). Nevertheless, most of the reported pathogen hosts and pathogenicity studies of Pseudoseptoria species are not recent. Thus, further studies are necessary to understand Pseudoseptoria hosts and their pathogenicity.
Type species: Pseudoseptoria donacis (Pass.) B. Sutton,
For other species: (Species Fungorum – search Pseudoseptoria)
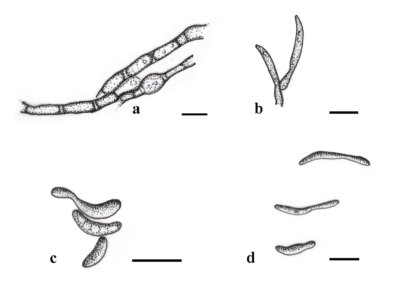
Figure 1 – Pseudoseptoria obscura. a Chlamydospore-like cells developing. b Conidiogenous cells. c, d Conidia. Scale bars: a-d = 10 μm. Redrawn from Quaedvlieg et al. (2013).
References
Spegazzini C. 1910 – Mycetes argentinenses, series V. An Mus Nac Hist Nat B Aires, 20, 329–467.
Entry by
Herbert Dustin R. Aumentado, Center of Excellence in Fungal Research and School of Science, Mae Fah Luang University, Chiang Rai, Thailand
(Edited by Ruvishika S. Jayawardena, Kevin D. Hyde, Samaneh Chaharmiri-Dokhaharani, & Achala R. Rathnayaka)
Published online 20 May 2024