Fungalpedia – Note 110 Coniella
Coniella Höhn.
Citation when using the entry: Senanayake et al., in prep – Diaporthomycetidae. Mycosphere.
Index Fungorum, Facesoffungi, MycoBank, GenBank, Fig. 1.
Coniella was introduced by Höhnel (1918) with the type species Coniella pulchella Höhn. Petrak & Sydow (1927) divided the genus into two subgenera, Euconiella and Pseudoconiella, and introduced another asexual genus for Schizoparme Shear, and Pilidiella Petr. & Syd. Following the proposal of Castlebury et al. (2002), Rossman et al. (2007) transferred the genus from Melanconidiaceae to the newly introduced family Schizoparmaceae in Diaporthales. Coniella has been subjected to several comprehensive taxonomic refinements by several morpho-molecular studies (Castlebury et al. 2002, Van Niekerk et al. 2004, Rossman et al. 2007, Alvarez et al. 2016). Alvarez et al. (2016) synonymized both Pilidiella and Schizoparme under Coniella, and the latter was accepted as the only genus in Schizoparmaceae. Members of Coniella are commonly found in different habitats including soil, decaying plant material, and endophytes within living plant tissues (Castlebury et al. 2002, Van Niekerk et al. 2004, Alvarez et al. 2016, Chethana et al. 2017). This asexual genus exhibits a wide range of morphological characteristics, with their ostiolate conidiomata typically being pycnidial, immersed to semi-immersed, subepidermal, erumpent, unilocular, brown to dark brown or black, and globose or slightly depressed globose to subglobose. The conidiophores may be formed singly or in clusters, simple to branched at the base, and septate. Percurrently proliferating conidiogenous cells are hyaline, subcylindrical, obclavate to lageniform and of annellidic or phialidic origin. The conidia are often unicellular, ellipsoidal, globose, and smooth with a truncate base and obtuse to apiculate apex. The sexual morph of Coniella is characterized by solitary, scattered, subepidermal, erumpent to superficial, perithecial, globose, coriaceous, and papillate ascomata with a periphysate ostiole. Asci are 8-spored, unitunicate, sessile, clavate to subcylindrical, with an apical ring, with biseriate, ellipsoidal, aseptate ascospores that are initially hyaline, becoming pale brown at maturity, with or without mucoid caps (Van Niekerk et al. 2004, Alvarez et al. 2016). They are cosmopolitan in distribution and known for their ecological significance (Van Niekerk et al. 2004, Mirabolfathy et al. 2012, Alvarez et al. 2016, Chethana et al. 2017, Raudabaugh et al. 2018). Some species are saprobic, playing a crucial role in decomposing organic matter and nutrient recycling (Raudabaugh et al. 2018). Other Coniella species are often associated with foliar, fruit, stem and root diseases in a wide range of hosts, such as grape white rot caused by Coniella diplodiella (Speg.) Petr. & Syd. and C. vitis Chethana, JY. Yan, XH. Li & KD. Hyde (Chethana et al. 2017), strawberry fruit and leaf diseases caused by C. castaneicola (Ellis & Everh.) B. Sutton (Grantina-Ievina & Kalniņa 2016), and pomegranate cankers, crown rots, dieback, fruit rots, leaf spots, shoot blights, and twig blights caused by C. granati (Sacc.) Petr. & Syd. (Mirabolfathy et al. 2012, Chen et al. 2014). Alvarez et al. (2016) and Chethana et al. (2017) adapted a morphology combined with multi-gene phylogeny and Genealogical Concordance Phylogenetic Species Recognition (GCPSR) for defining species boundaries in Coniella. Recommended genetic markers for the genus include ITS, LSU, tef1-α, rpb2 and histone (Alvarez et al. 2016, Chethana et al. 2017). Sixty-four species epithets are listed for Coniella in Index Fungorum (2023), of which 38 have been accepted based on morpho-molecular approaches.
Type species: Coniella pulchella Höhn.
Other accepted species: see Species Fungorum, search Coniella for names.
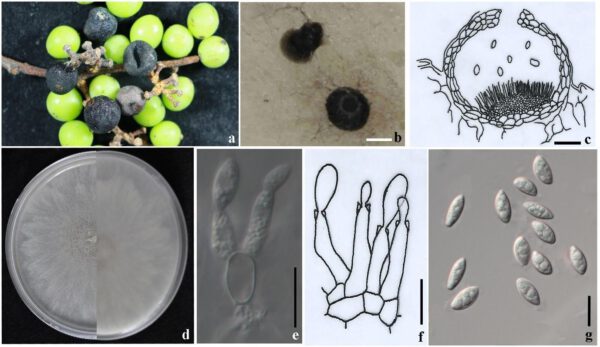
Figure 1 – Coniella vitis (MFLUCC 16-1404). a Conidiomata on diseased fruits, b Conidiomata on PDA, c Cross section of a conidioma (re-illustrated from Van Niekerk et al. 2004), d Upper (left) and reverse (right) views of the colony on the PDA, e, f Conidiophores and conidiogenous cells, g Conidia. Scale bars: b = 100 μm, c, e-g = 10 μm.
References
Alvarez LV, Groenewald JZ, Crous PW. 2016 – Revising the Schizoparmaceae: Coniella and its synonyms Pilidiella and Schizoparme. Studies in Mycology 85, 1–34.
Castlebury LA, Rossman AY, Jaklitsch WJ et al. 2002 – A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94, 1017–1031.
Chen Y, Shao DD, Zhang AF et al. 2014 – First report of a fruit rot and twig blight on pomegranate (Punica granatum) caused by Pilidiella granati in Anhui Province of China. Plant Disease 98, 695.
Mirabolfathy M, Groenewald JZ, Crous PW. 2012 – First report of Pilidiella granati causing dieback and fruit rot of pomegranate (Punica granatum) in Iran. Plant Disease 96, 461.
Raudabaugh DB, Iturriaga T, Carver A, Mondo S et al. 2018 – Coniella lustricola, a new species from submerged detritus. Mycological Progress 17, 191–203.
Entry by
Chethana K.W.T, Center of Excellence in Fungal Research and School of Science, Mae Fah Luang University, Chiang Rai, Thailand
(Edited by Kevin D. Hyde)
Published online 22 September 2023