Fungalpedia – Note 130, Boeremia
Boeremia Aveskamp, Gruyter & Verkley
Citation when using this entry: Aumentado et al. 2024 (in prep) – Fungalpedia, plant pathogens.
Index Fungorum, Facesoffungi, MycoBank, GenBank, Fig 1.
Classification: Didymellaceae, Pleosporales, Pleosporomycetidae, Dothideomycetes, Pezizomycotina, Ascomycota, Fungi
Boeremia, introduced by Aveskamp et al. (2010), was established to include phoma-like species that share morphological similarities with Phoma exigua. Boeremia is typically characterised by variable-shape and -size pycnidial conidiomata, mostly globose to subglobose, smooth or with few mycelial outgrowths on the agar surface or immersed. Pycnidia can be solitary or confluent, with 1–2(–3) ostioles that are apapillate or papillate. When mature, ostioles are lined internally with papillate hyaline cells. The pycnidial wall consists of pseudoparenchymatous tissue, composed of 2–8 cell layers, with the outer 1–3 layers being brown-pigmented (Aveskamp et al. 2010, Jayawardena et al. 2019). The conidiogenous cells are phialidic, hyaline, simple, smooth, ampulliform to doliiform. The conidia vary in shape and are hyaline, thin-walled, smooth, and mainly aseptate; however, occasionally, 1(–2)-septate conidia may be found. Pseudothecia are only rarely observed in one species in vivo, and they are subglobose. The asci are cylindrical or subclavate, always 8-spored and biseriate, whereas the ascospores are ellipsoid and uniseptate (Jayawardena et al. 2019, Jayasiri et al. 2017, Aveskamp et al. 2010, Boerema 2004).
This genus is accepted in Didymellaceae (Hongsanan et al. 2020, Wijayawardene et al. 2022). Boeremia can be distinguished from other genera in Didymellaceae based on the morphology of its ostiole, which has a smooth lining and distinct hyaline cells surrounding the ostiolar openings. Moreover, these species produce fewer conidia in culture than the host (Aveskamp et al. 2010). There are 22 species associated with Boeremia, identified through a combination of morpho-molecular sequence data of recommended genetic markers, ITS, LSU, rpb2, tub2, and tef1-α (Jayawardena et al. 2019, Jayasiri et al. 2017, Marin-Felix et al. 2017, Chen et al. 2015, 2017, Aveskamp et al. 2010, Berner et al. 2015).
Boeremia is a ubiquitous necrotrophic plant pathogen that affects all foliar parts of the plant (Berner et al. 2015, You et al. 2016, Zhao et al. 2016, Gai et al. 2016, Grinbergs et al. 2014, 2016). It causes dark brown sunken lesions at the base of the plant, which eventually expand to girdle the stem, resulting in yellowing and wilting of older leaves, ultimately leading to the death of the plant. Fruit infection begins as water-soaked lesions that rapidly progress into sunken brown/black/grey lesions with concentric rings. Leaf lesions, on the other hand, start as small spots and develop into brown/grey lesions with concentric rings (Zhao et al. 2016, Jones et al. 2011). Pathogenicity studies have been conducted in Abelmoschus esculentus (Zhao et al. 2016), Coffea arabica (Núñez et al. 2011), Rhaponticum repens (Berner et al. 2015), Phaseolus spp. (Gorny et al. 2015, Ríos et al. 2014, Li et al. 2012), and Pyrethrum sp. (Zhao et al. 2016) confirming its pathogenicity on the respective hosts. A study by Berner et al. (2015) on host range, disease incidence, and severity of B. rhapontica demonstrated its capacity to infect is confined to a very specific range of hosts within the Rhaponticum group. In contrast, other weed species that were examined resulted in a 20% severity (Berner et al. 2015, Hidalgo et al. 2006). However, B. exigua has been documented to cause leaf spot Panax japonicus infecting approximately 95% of plants in the field, resulting in substantial necrotic lesions on leaves with elliptical and irregular margins at the leaf tip (You et al. 2016). Another study of B. exigua on Cichorium intybus induced dark, firm, sunken lesions on the root. As the disease progresses, these lesions transform into cavities that are black on the crown of the plant resulting in a yield reduction of up to 31% (Grinbergs et al. 2014, 2016).
Boeremia also can be found as saprobes on various plant species including Chamaedaphne calyculata, Cheiranthus cheiri, Crinum powellii, Cynara scolymus, Dactylis purpurea, Dahlia sp., Digitalis sp., Foeniculum vulgare, Forsythia sp., Fraxinus excelsior, Hedera helix, Hydrangea paniculata, Galium sp., Ipomoea batatas, Lamium maculatum, Lathyrus sp., Leonurus cardiaca, Linum usitatissimum, Lonicera sp., Lycopersicon esculentum, Malus domestica, Melissa officinalis, Mentha sp., Nemophila insignis, Nerium oleander, Nicotiana tabacum, Origanum dubium, Oxycoccus macrocarpus, Philadelphus sp., Phlox sp., Pisum sativum, Populus euramericana, Salix sp., Sambucus nigra, Sedum spp., Solanum tuberosum, Syringa vulgaris, Tanacetum cinerariifolium, Trachelospermum jasminoides, Ulmus sp., Veronica officinalis, Viburnum opulus, and Vitis sp. (Farr & Rossman 2023).
Type species: Boeremia exigua (Desm.) Aveskamp, Gruyter & Verkley
For other species: Species Fungorum, search Boeremia
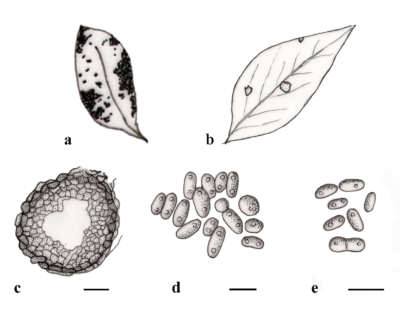
Figure 1 – Boeremia exigua. a, b. Symptoms on leaves. c Section of pycnidium. d, e Conidia. Scale bars: c = 20μm; d = 5 μm; e = 10 μm. Redrawn from Aveskamp et al. (2010) and Chen et al. (2015).
References
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM et al. 2010 – Highlights of the Didymellaceae: a polyphasic approach to characterise Phoma and related pleosporalean genera. Studies in Mycology 65(1), 1–60.
Berner D, Cavin C, Woudenberg JH, Tunali B et al. 2015 – Assessment of Boeremia exigua var. rhapontica, as a biological control agent of Russian knapweed (Rhaponticum repens). Biological Control 81, 65–75.
Boerema GH ed., 2004 – Phoma identification manual: differentiation of specific and infra-specific taxa in culture. CABI.
Chen Q, Jiang JR, Zhang GZ, Cai L et al. 2015 – Resolving the Phoma enigma. Studies in mycology 82, 137–217.
Chen Q, Hou LW, Duan WJ, Crous PW et al. 2017 – Didymellaceae revisited. Studies in Mycology 87(1), 105–159.
Farr DF Rossman AY. 2023 – Fungal databases. U.S. National Fungus Collections, ARS, USDA. https://nt.ars-grin.gov/fungaldatabases/
Gai YP, Ma HJ, Chen XL, Chen HH et al. 2016 – Boeremia tuber rot of sweet potato caused by B. exigua, a new post-harvest storage disease in China. Canadian Journal of Plant Pathology 38(2), 243–249.
Grinbergs DE, France RA. 2014 – Black root rot of industrial chicory (Cichorium intybus L. var. sativum) in Chile caused by Boeremia exigua var. exigua. Phytopathology 104(11), 47.
Grinbergs D, France A, Varrelmann M. 2016 – First Report of Boeremia exigua var. exigua (syn. Phoma exigua var. exigua) causing black root rot on Industrial Chicory (Cichorium intybus var. sativum) in Chile. Plant Disease 100(11), 2328.
Gorny AM, Kikkert JR, Dunn AR, Dillard HR et al. 2015 – Tan spot of lima bean caused by Boeremia exigua var. exigua in New York State, USA. Canadian Journal of Plant Pathology 37(4), 523–528.
Hidalgo O, Garcia-Jacas N, Garnatje T, Susanna A. 2006 – Phylogeny of Rhaponticum (Asteraceae, Cardueae–Centaureinae) and related genera inferred from nuclear and chloroplast DNA sequence data: taxonomic and biogeographic implications. Annals of Botany 97(5), 705–14.
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN et al. 2020 – Refined families of Dothideomycetes: Orders and families incertae sedis in Dothideomycetes. Fungal Diversity 105, 17–318.
Jayasiri SC, Hyde KD, Jones EBG, Jeewon R et al. 2017 – Taxonomy and multigene phylogenetic evaluation of novel species in Boeremia and Epicoccum with new records of Ascochyta and Didymella (Didymellaceae). Mycosphere 8(8), 1080–1101.
Jayawardena RS, Hyde, KD, Jeewon R, Ghobad-Nejhad M et al. 2019 – One stop shop II: taxonomic update with molecular phylogeny for important phytopathogenic genera: 26–50. Fungal Diversity 94, 41–129.
Jones SJ, Hay FS, Harrington TC, Pethybridge SJ. 2011 – First report of Boeremia blight caused by Boeremia exigua var. exigua on pyrethrum in Australia. Plant Disease 95(11), 1478–1478.
Li YP, You MP, Finnegan PM, Khan TN et al. 2012 – First report of black spot caused by Boeremia exigua var. exigua on field pea in Australia. Plant Disease 96(1), 148–148.
Ligarreto G, Gutiérrez LNG, Ladino CCP. 2023 – Differential responses of Phaseolus spp. against Black node disease (Boeremia noackiana). Bragantia 82, e20220225.
Marin-Felix Y, Groenewald JZ, Cai L, Chen Q et al. 2017 – Genera of phytopathogenic fungi: GOPHY 1. Studies in mycology. 86, 99–216.
Núñez M, Mejía L. 2021 – First report of Boeremia exigua, as a pathogen causing Melt disease, in coffee plantations (Coffea arabica variety geisha) in the highlands of Panama. In National Congress of Science and Technology–APANAC 315–322.
Ríos MDK, Viteri RSE, Delgado HH 2014 – Agronomic evaluation of advanced common climbing bean Phaseolus vulgaris L. lines in Paipa, Boyaca. Revista de Ciencias Agrícolas 31(1), 42–54.
You JM, Wang QH, Wang GJ, Lin XM et al. 2016 – First report of Phoma exigua causing leaf spot on Japanese ginseng (Panax japonicus) in China. Plant Disease 100(2), 534.
Zhao Q, Xie XW, Shi YX, Chai AL, et al. 2016 – Boeremia leaf and fruit spot of okra caused by Boeremia exigua in China. Canadian Journal of Plant Pathology 38(3), 395–399.
Entry by
Herbert Dustin R. Aumentado, Center of Excellence in Fungal Research and School of Science, Mae Fah Luang University, Chiang Rai, Thailand
(Edited by Ruvishika S. Jayawardena, Kevin D. Hyde, Samaneh Chaharmiri-Dokhahrani, & Achala R. Rathnayaka)
Published online 5 September 2023