Fungalpedia – Note259, Paraphoma
Paraphoma Morgan-Jones & J.F. White.
Citation when using this entry: Aumentado et al. 2024 (in prep) – Fungalpedia, plant pathogens.
Index Fungorum, Facesoffungi, MycoBank, GenBank, Fig. 1.
Classification: Phaeosphaeriaceae, Pleosporales, Pleosporomycetidae, Dothideomycetes, Pezizomycotina, Ascomycota, Fungi
Paraphoma is a genus classified under Phaeosphaeriaceae, first introduced by Morgan-Jones et al. (1983) and typified by Paraphoma radicina (de Gruyter & Boerema 2002, de Gruyter et al. 2010). Paraphoma is characterised by solitary or aggregated pycnidial conidiomata that can be superficial or semi-immersed, brown to dark brown, globose to subglobose, with a short neck, uniloculate, and setose. Ostiole is singular and round. The pycnidium wall is thick and consists of an outer layer 2 cells thick, made of dark brown cells, and an inner layer, 4-5 cells thick, composed of subhyaline to pale brown pseudoparenchymatous cells. The pycnidial setae are copious, straight, or slightly flexuous, acuminate, and can be smooth or verrucose. They have thick walls, are septate, and have acuminate, subacute to acute, and pale brown to subhyaline apices. Micropycnidia may be fertile or sterile and are abundantly produced in some Paraphoma species when submerged in the medium. Conidiogenous cells are monophialidic and are formed from the inner cells of the pycnidial wall, hyaline to subhyaline, discrete, determinate, and lageniform. Conidiophores are ampulliform and mostly reduced to phialidic conidiogenous cells. The presence of chlamydospores varies depending on the species; they may be absent or present, solitary, short or long chains, or aggregated. Multicellular chlamydospores can be alternarioid, pseudosclerotioid, epicoccoid, or botryoid depending on the species. The conidia are enteroblastic, ellipsoid, aseptate, hyaline, smooth, and guttulate (Morgan-Jones et al. 1983, Marin-Felix et al. 2019).
Previous studies have demonstrated that P. radicina forms a distinct group outside Didymellaceae, leading to its exclusion from Phoma (de Gruyter et al. 2013). Moreover, in a more recent phylogenetic analysis based on LSU and SSU, P. radicina clustered within Phaeosphaeriaceae, while other species were found in Cucurbitariaceae and Coniothyriaceae. Thus, to better delineate Paraphoma, phylogenetic studies based on the ITS, LSU, in combination with protein-coding genes β-tub, tef1α, and rpb2, have been recommended (de Gruyter et al. 2010, Moslemi et al. 2016, 2018, Crous et al. 2017, 2021, Marin-Felix et al. 2019, Gomzhina et al. 2020, Magaña-Dueñas et al. 2021, Guarnaccia et al. 2022). There are 15 recognised species of Paraphoma based on morpho-molecular analyses (Species Fungorum 2024).
Most Paraphoma species are soil-borne and foliar pathogens that cause crown discoloration, root rot, and necrotic leaf spots on herbaceous plants. They have a wide host range that includes monocotyledonous plants, Asteraceae, Cupressaceae, Rosaceae, and Solanaceae (Marin-Felix et al. 2019). Among these are recent reports are P. radicina causing root rot of Medicago sativa which demonstrated in pathogenicity tests had substantial damage to Medicago sativa (Cao et al. 2022, Dang et al. 2022). Pathogenicity tests were also conducted on P. chrysanthemicola causing crown and leaf rot in Atractylodes lancea, which exhibited leaf rot symptoms, including lesions, yellowing, and withering (Wang et al. 2022), P. vinacea causing crown rot of Pyrethrum (Moslemi et al. 2016, 2018), and P. garibaldii cspotsng leaf spot of Campanula rapunculoides (Guarnaccia et al. 2022).
Type species: Paraphoma radicina (McAlpine) Morgan-Jones & J.F. White
Other species: (Species Fungorum – search Paraphoma)
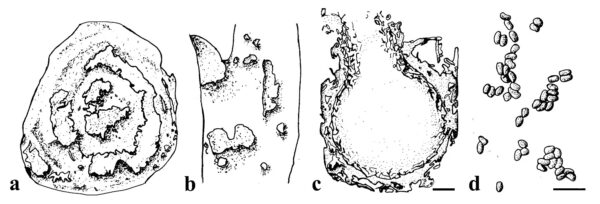
Figure 1 – Paraphoma spp. a Crown discolouration caused by Paraphoma vinacea. b Water-soaked and necrotic leaf lesions caused by Paraphoma chlamydocopiosa. c Conidiomata of Paraphoma vinacea. d Conidia. Scale bars: c = 100 µm; d = 10 µm. Redrawn from (Moslemi et al. 2016, 2018, Marin-Felix et al. 2019).
References
Guarnaccia V, Martino I, Tabone G, Crous PW et al. 2022 – Paraphoma garibaldii sp. nov. causing leaf spot disease of Campanula rapunculoides in Italy. Fungal Systematics and Evolution 9(1), 19–26.
Morgan-Jones G, White JF. 1983 – Studies in the genus Phoma. III. Paraphoma, a new genus to accommodate Phoma radicina [Fungi]. Mycotaxon (USA).
Wang HY, Kang CZ, Wang YF, Wang S et al. 2022 – First report of Paraphoma chrysanthemicola causing crown and leaf rot of Atractylodes lancea in China. Plant Disease 106(8), 2268.
Entry by
Herbert Dustin R. Aumentado, Center of Excellence in Fungal Research and School of Science, Mae Fah Luang University, Chiang Rai, Thailand
(Edited by Ruvishika S. Jayawardena, Kevin D. Hyde, Samaneh Chaharmiri-Dokhaharani, & Achala R. Rathnayaka)
Published online 21 May 2024