Fungalpedia – Note 257, Cryphonectria
Cryphonectria (Sacc.) Sacc. & D. Sacc.
Citation when using this entry: Aumentado et al. 2024 (in prep) – Fungalpedia, plant pathogens.
Index Fungorum, Facesoffungi, MycoBank, GenBank, Fig 1.
Classification: Cryphonectriaceae, Diaporthales, Diaporthomycetidae, Sordariomycetes, Pezizomycotina, Ascomycota, Fungi
Cryphonectria belongs to Cryphonectriaceae (Diaporthales) (Gryzenhout et al. 2006a, 2006b), and was established to incorporate Nectria abscondita and N. variicolor, although the type species were not specified (Saccardo & Saccardo 1905, Jiang et al. 2018). Hence, Gryzenhout et al. (2005) proposed Cryphonectria parasitica as the type species of Cryphonectria, which is known to cause chestnut blight. The defining sexual characteristics of Cryphonectria include clustered or solitary, partially embedded, protruding orange ascostromata. The ascostromata are cylindrical-fusoid to club-shaped, attached to pedicels, and contain eight-spored asci exhibiting distinctive refractive J-shaped rings. The ascospores are elliptical, spindle-shaped, cylindrical, aseptate, typically transparent but occasionally brown (Jiang et al. 2020). The asexual morph of Cryphonectria is a pear-shaped, spherical, or flattened loculate conidiomata. The conidiophores are cylindrical with swollen bases and produce flask-shaped conidiogenous cells and cylindrical conidia that are non-segmented, transparent, and typically elongated (Jiang et al. 2020).
Species Fungorum (2024) listed 26 Cryphonectria epithets. However, with the synonymisation of Cryphonectria decipiens and Cryphonectria naterciae (Rigling & Prospero 2018) and Cryphonectria japonica and Cryphonectria nitschkei (Senanayake et al. 2023), 12 species have been accepted based on morphology and molecular analysis utilising concatenated LSU, ITS, rpb2, β-tubulin (T1/T2), and tef1-α gene regions (Gryzenhout et al. 2009, Braganca et al. 2011, Jiang et al. 2018, 2019, 2020, Senanayake et al. 2023).
Cryphonectria is a hemibiotrophic fungus that includes various important species that cause devastating diseases, notably, C. parasitica is a prominent invasive pathogen that incites necrotic spots (cankers) on the bark of trees (Rigling & Prospero 2018, Stauber et al. 2020). Cryphonectria infect parts of trees above the ground, such as stems, branches, and twigs. The disease shows symptoms of longitudinal splits along the bark (Diller 1965) and light brown mycelial fans (Rigling & Prospero 2018). This ultimately causes wilting in the affected parts of the plant. Cryphonectria infection also induces in production of new shoots below the canker. Less harmful cankers on susceptible host trees are typically linked to mycovirus-induced hypovirulence, resulting in non-lethal, surface-level or callused cankers. Cryphonectria spp. infect various plant hosts including Acer spp. (Aceraceae), Carpinus betulus (Betulaceae), Castanea crenata, C. dentata, C. mollissima, C. pumila, C. sativa (Fagaceae), and Quercus spp. (Fagaceae) (Rigling and Prospero 2018, Dennert et al. 2020). A recent genomic study of Cryphonectria revealed that GH28 genes are responsible for pectin breakdown in C. parasitica. The loss of CAZymes might have boosted the ability of C. parasitica to cause disease in Castanea species (Sprockett et al. 2011, Stauber et al. 2020). Pathogenicity studies demonstrated that C. japonica caused less severe disease than C. parasitica (Dennert et al. 2020) and C. parasitica caused a 52-88 % decline in Castanea sativa and Quercus petraea (Karadžić et al. 2019).
Type species: Cryphonectria parasitica D. Sacc.
Other species: (Species Fungorum – search Cryphonectria)
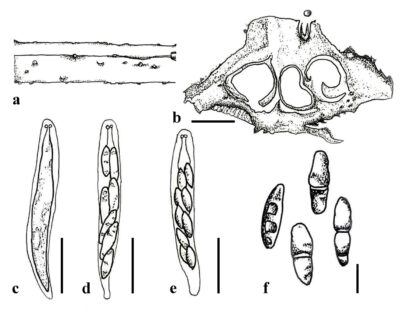
Figure 1 – Cryphonectria kunmingensis. a Ascostromata on the host surface. d Cross section of ascostromata. c–e Asci. f Ascospores. Scale bars: b = 300 µm, c–e = 20 µm, f = 5 µm. Redrawn from Senanayake et al. (2023).
References
Saccardo PA, Saccardo D. 1905 – Sylloge fungorum. Patavii 17, 783–784.
Species Fungorum 2023 – Accessed on November 20, 2023, at URL: https://speciesfungorum.org/.
Entry by
Herbert Dustin R. Aumentado, Center of Excellence in Fungal Research and School of Science, Mae Fah Luang University, Chiang Rai, Thailand
(Edited by Ruvishika S. Jayawardena, Kevin D. Hyde, Samaneh Chaharmiri-Dokhaharani, & Achala R. Rathnayaka)
Published online 21 May 2024