Fungalpedia – Note 66 Allantophomopsiella
Allantophomopsiella Crous
Citation when using this data: Huanraluek et al. Fungalpedia, coelomycetes. Mycosphere (in prep).
Index Fungorum, Facesoffungi, MycoBank, GenBank, Coelomycetes.org, Fig 1.
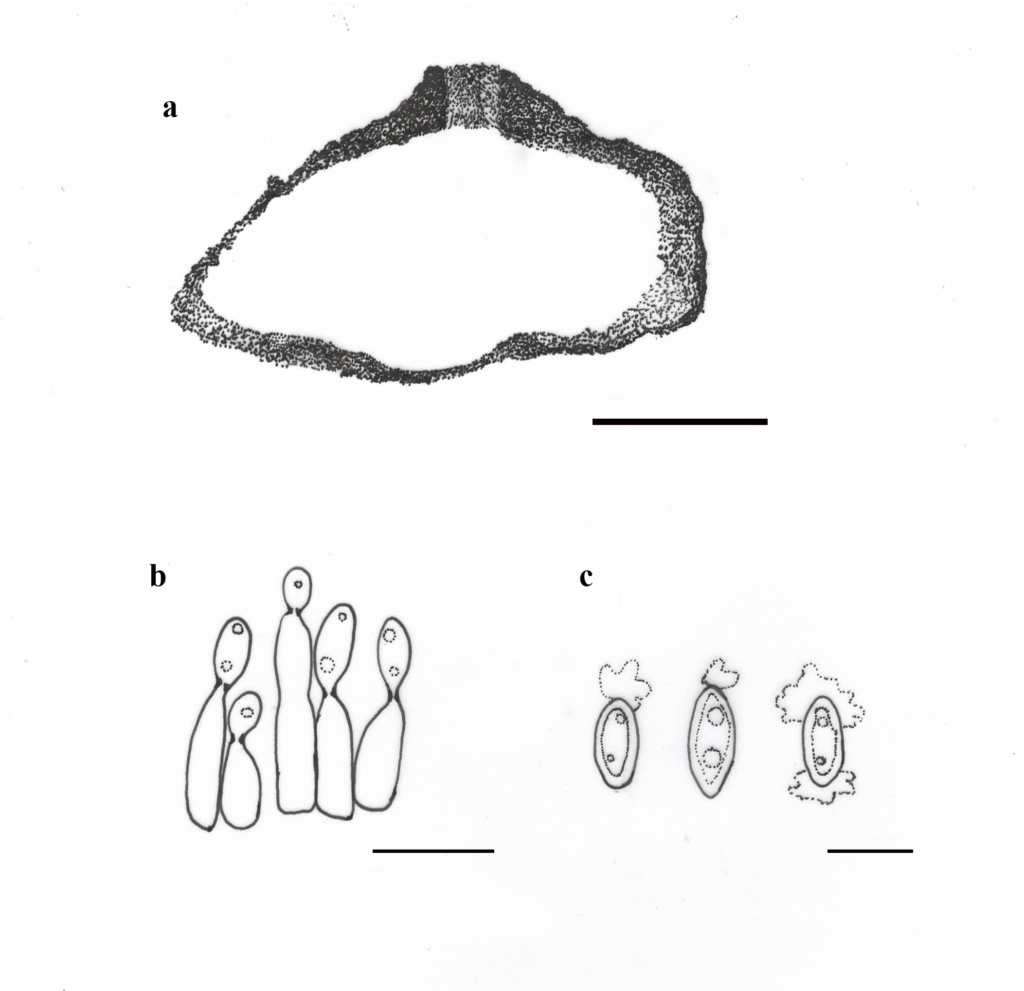
Allantophomopsiella was described based on ITS and LSU sequence data and was transferred to Leotiomycetes (Phacidiaceae, Phacidiales, Leotiomycetidae) (Li et al. 2020). Allantophomopsiella is a monotypic genus (https://www.indexfungorum.org/Names/Names.asp on 18 May 2023). Conidiomata of this genus are pycnidial, immersed, septate conidiophores arise from the inner layer of the conidiomata, conidiogenous cells are integrated or discrete, ampulliform to subcylindrical or lageniform, hyaline and conidia are ellipsoid to fusiform, hyaline, smooth, aseptate, guttulate, bearing mucoid apical appendages, flabelliform to irregular in shape (Crous et al. 2014, Li et al. 2020). This genus is distributed in Austria, Canada, Germany, Latvia, Sweden, the UK, and the USA (Crous et al. 2015, Li et al. 2020). Allantophomopsiella is morphologically related to the phylogenetic genera Apostasseria and Allantophomopsis. However, distinct from lacking percurrent proliferation on conidiogenous cell and having inequilaterally fusiform or naviculate conidia (Crous et al. 2014, Marin-Felix et al. 2019). Crous et al. (2014) reported that A. pseudotsugae is a pathogen of conifers (Roll Hansen 1992), that was found to be very damaging, especially after wounding tree dormancy (Marin-Felix et al. 2019).
Type species: Allantophomopsiella pseudotsugae (M. Wilson) Crous 2014.
Fig 1 – Allantophomopsiella pseudotsugae (redrawn from Crous et al. 2014, Li et al. 2020) a Vertical section of conidioma. b Conidiogenous cells giving rise to conidia. c Conidia. Scale bars: a = 50 µm, b = 10 µm, c-e = 5 µm.
References
Crous PW, Carris LM, Giraldo A, Groenewald JZ, et al. 2015– The Genera of Fungi – fixing the application of the type species of generic names -G 2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia, IMA fungus 6, 163–98. https://doi.org/10.5598/imafungus.2015.06.01.11
Crous PW, Quaedvlieg W, Hansen K, Hawksworth DL, et al. 2014– Phacidium and Ceuthospora (Phacidiaceae) are congeneric: taxonomic and nomenclatural implications. IMA fungus 5, 173–193. https://doi.org/10.5598/imafungus.2014.05.02.02
Li WJ, McKenZie EHC, Liu JK, Bhat DJ, et al. 2020– Taxonomy and phylogeny of hyaline-spored coelomycetes. Fungal Diversity 100, 279–801. https://doi.org/10.1007/s13225-020-00440-y
Marin-Felix Y, Hernández-Restrepo M, Wingfield MJ, Akulov A, et al. 2019–Genera of phytopathogenic fungi: GOPHY 2. Studies in Mycology 92, 47–133. https://doi.org/10.1016/j.simyco.2018.04.002
Roll Hansen F. 1992– Important pathogenic fungi on conifers in Iceland. Acta Botanica Islandica 11, 9–12. https://utgafa.ni.is/Acta-Botanica-Islandica/Acta-Botanica-Islandica-11/Acta-Botanica-Islandica-11-2.pdf
Entry by
Naruemon Huanraluek, Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, Thailand.
(Edited by Kevin D Hyde & Ruvishika S. Jayawardena)